FDA approves Gilead’s Remdesivir as coronavirus treatment
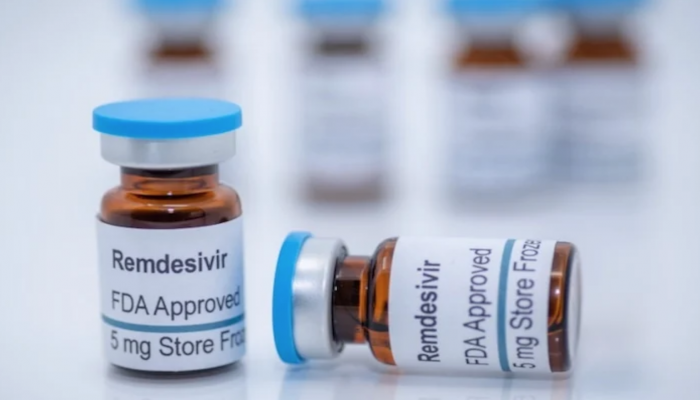
Shafaq news/ (CNBC) The Food and Drug Administration on Thursday approved Gilead Sciences’ antiviral drug Remdesivir as a treatment for the coronavirus.
In May, the FDA granted the drug an emergency use authorization, allowing hospitals and doctors to use it on patients hospitalized with the disease even though the medication had not been formally approved by the agency. The intravenous drug has helped shorten the recovery time of some hospitalized Covid-19 patients. It was one of the drugs used to treat President Donald Trump, who tested positive for the virus earlier this month.
The drug will be used for Covid-19 patients at least 12 years old and requiring hospitalization, Gilead said. Remdesivir is now the first and only fully approved treatment in the U.S. for Covid-19, which has infected more than 41.3 million people worldwide and killed more than 1 million, according to data compiled by Johns Hopkins University.
“Since the beginning of the COVID-19 pandemic, Gilead has worked relentlessly to help find solutions to this global health crisis,” Gilead CEO Daniel O’Day said in a statement. “It is incredible to be in the position today, less than one year since the earliest case reports of the disease now known as COVID-19, of having an FDA-approved treatment in the U.S. that is available for all appropriate patients in need.”
Remdesivir is approved or authorized for temporary use as a Covid-19 treatment in approximately 50 countries worldwide, according to the company.
Earlier this month, a study coordinated by the World Health Organization had indicated that the drug had “little or no effect” on death rates among hospitalized patients. Still, it has shown to be modestly effective in reducing the recovery time for some hospitalized patients.
Earlier in the year, Dr. Anthony Fauci, the nation’s leading infectious disease expert, said the drug would set “a new standard of care” for Covid-19 patients.
The majority of patients treated with Remdesivir receive a five-day course using six vials of the drug. The company is also developing an inhaled version of the medication, which it will administer through a nebulizer, a delivery device that can turn liquid medicines into mist. The company has said the drug can’t be administered in pill form because its chemical makeup would impact the liver.
Remdesivir, now under the brand name Veklury, costs $2,340 for a five-day treatment course for people covered by government health programs and other countries’ health-care systems, and $3,120 for U.S. patients with private health coverage.
In August, the company said it planned to produce more than 2 million treatment courses of Remdesivir by the end of the year and anticipated being able to make “several million more” in 2021, adding it has increased the supply of the drug more than fiftyfold since January. Its manufacturing network now includes more than 40 companies in North America, Europe and Asia.