A pharmaceutical company in Erbil dismantles COVID-19 and gives hope to Iraq and neighboring countries

Shafaq News / Amid the darkness accompanied COVID-19 pandemic and its spread all over the world, a glimmer of hope sparked in Erbil, where a company is working day and night to produce a drug -that has proven its efficacy globally- and provide it to Iraqis, as well as other countries suffering from the pandemic in the region.
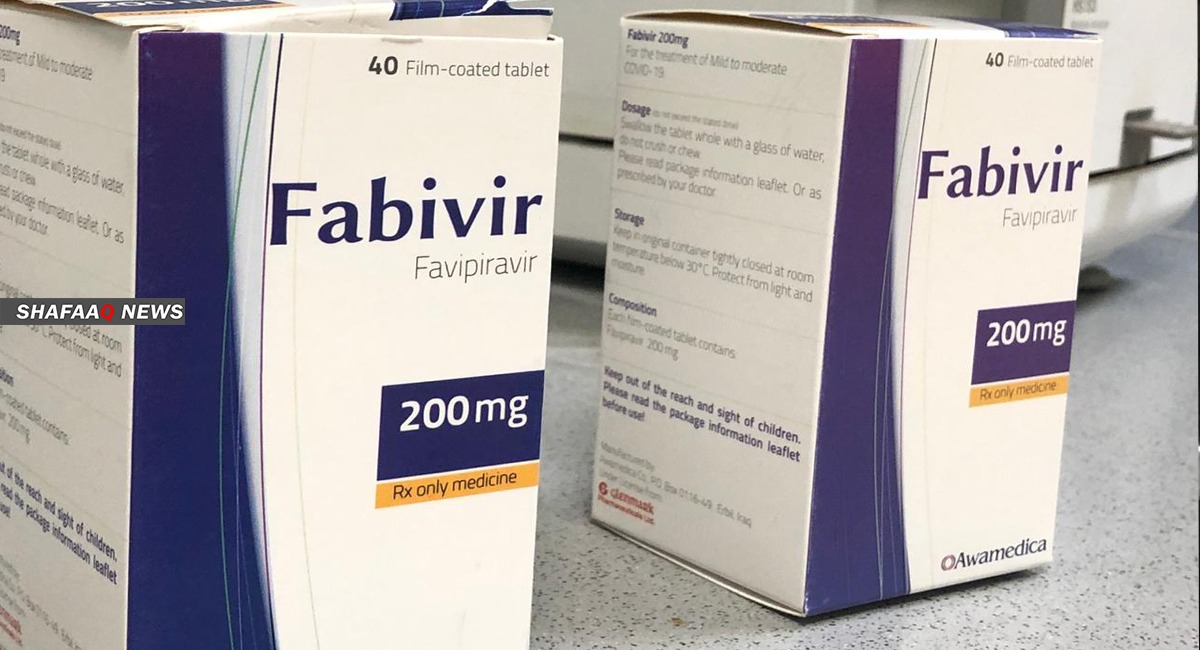
Awamedica company is doing an important job, as it had carried out experiments and studies and moved to the process of manufacturing the drug "Favipiravir"; in the absence of a promising alternative drug so far, not only in Iraq but in the whole world, which makes this drug a real spark of optimism for Kurdistan and the rest of the Iraqi provinces.
Shafaq News agency interviewed Haidar Zaki Shuker, a pharmacist who works as assistant director of the pharmacist in charge of Awamedica Pharmaceuticals, to follow up the development of this drug, which has become common in Japan, China, India, Russia, and other countries; and the Iraqi government has adopted it as a successful drug to treat Covid-19 disease.
There is an ongoing debate about the drug; its price, whether it will be accessible to Iraqis in general, and whether its prices will be manipulated according to the needs of citizens. Taking into consideration Awamedica pharmaceuticals (based in Erbil) -in its agreement with the international company that manufactures the drug- is authorized to distribute Favipiravir to five regional countries: Syria, Yemen, Lebanon, Iran and Azerbaijan.
Responding to a question regarding Favipiravir’s mechanism of action; Haidar Shuker told Shafaq News agency, “It is an antiviral that encapsulates the virus and reduces its spread in the body, since the moment it turns into a living organism, it expands and hits more than one organ: The respiratory system, the intestines…etc. By taking it on the onset of the infection, the stimulation, activity, reproduction, symptoms and transmission of the virus will be reduced”.
"tracking the recovery rates; the global success rate of patients who have responded to treatment is 80%-90%. There are Russian reports of 90% in four days, Indian reports of 85% and Chinese of 75%", Shuker revealed.
Shuker answered a question about the patients’ response to the drug in Iraq, "In fact, the response is very good and a whole team of our company is following up on the topic; in the first stage we were following the side effects -which are non-existent or minor -like the rest of the drugs. Most of those who used the drug showed a great response, as that they did not need oxygen supplementation or admission to the intensive care units (ICU)"
The drug is not new, according to Haidar Shuker, it was discovered in Japan in 2014 as a treatment for influenza that hit the country at that time. With the outbreak of COVİD-19, Japan readministered the drug to practice and it was very successful; then China and several countries redeveloped the treatment and put it into its therapeutic protocols, including India, Russia, and countries close to us today such as the UAE, KSA and Egypt. The Iraqi Ministry of Health then decided to integrate it into the therapeutic protocols.
Shuker explained that the rumors circulating in media about the drug being Russian is a fallacy; as it is originally Japanese and was produced later in China and India. Today it is produced in Iraq, Egypt, KSA and Turkey.
Regarding Awamedica, he pointed that it has been registered with the Ministry of Health in Baghdad since 2006, and has held international degrees and contracts for 14 years.
As for the company's relationship with COVİD-19, Shuker revealed that, "since the beginning of the pandemic, we thought -as a national factory- about means to participate in controlling it; We started with sterilizers and preventive equipment.. Until we got to the idea of "Favipiravir". Our company signed a contract with Glenmark pharmaceuticals -which has an American license (FDA)".
Cooperation was carried out between the research centers of the two parties; until an understanding was reached that Awamedica obtains the compounds of the drug from Glenmark to re-produce it in Iraq in the form of pills -after the Iraqi company studied it and assessed the response before its release to public use.
"We submitted all the studies related to this drug, which were conducted on patients in India, to Baghdad. Glenmark Company and the Iraqi Ministry of Health introduced the drug to the therapeutic protocol and approved it as a treatment for coronavirus (COVİD-19) and signed a contract with our company, which put it into production for the Ministry of Health", Haidar Shuker said.
About the policy of distributing the drug, Shuker said, "The Iraqi Ministry of Health has distributed it to health centers as well as COVİD-19 treatment centers. It will be distributed to all of Iraq; free of charge through the Ministry of Health. We will provide it to the private sector in Baghdad under certain controls related to counter-smuggling protocols, obligatory prescriptions and fixed prices to the patients; we are currently in contact with the Iraqi Ministry of Health about the mechanism of putting it in the private sector".
When asked about the distribution of the drug and whether it is limited to Kurdistan -whether it will include central and southern Iraq or even neighboring countries, he responded, “at the moment, there is a strategy to cover the requirements of the Iraqi Ministry of Health, as it has all the statistics for the patients. Afterward, in coordination with them, we will provide the private sector. if Iraq achieves self-sufficiency and we control the disease in the country, we give priority to other countries, although we received requests not to export the medicine until Iraq has reached local self-sufficiency", adding that, "The Ministry of Health has requested reinforcements of the medicine to cover all hospitals in the center and south of the country as well as Kurdistan".
The strategy also appears to be related to the level of production of the drug in the future. "Awamedica’s production capacity is very high. We can produce as much as 8,000-9,000 packages per day. According to the contract we have with Glenmark, we have the right to market the drug in five countries including Syria, Yemen, Lebanon, Iran and Azerbaijan".
The issue of debate in Iraq revolves around the company's pricing of the drug, especially in the private sector, and whether the company is seeking to exploit the poor situation of the healthcare system. In this context, Haidar Shuker clarified, "We have direct production price factors, as well as indirect ones. We usually calculate the cost of the drug by taking into account the expenses of the laboratory and the workers. However, this drug is new and expensive and the substance needs to be examined by accurate devices; These factors as well must be considered in the calculations".
He added, "Initially, we did not have any intention to put the medicine for trade in the private sector. Moreover, the price of our drug is 20% of similar but smuggled and non-official drugs -through Turkey and India. However, the Ministry of Health in Baghdad considered home quarantine and currently not all patients are admitted to government hospitals where this drug is distributed. Some patients need this drug, so in coordination with the Iraqi Ministry of Health, we will put it to the market and the price will be determined by the Ministry of Health in Baghdad".
Shuker continued, "The mechanism adopted is that when the material is registered, it is priced –the price should be certified by a committee. We didn't produce the medicine for trading and when you see the price you might consider it expensive, but the cost of the drug is like this and it costs a lot. In hospitals, it is distributed for free, just like other drugs in government hospitals".
When asked again by Shafaq News about the price of the drug he said, "the price will be announced when the drug is introduced to the private sector and will be published by our company. We call on citizens to not pay more than the price we set".
Health centers in Iraq started using Favipiravir, which is a sign of its success in managing the disease, Shuker said, "All hospitals, whether in Baghdad, central, and southern cities are using it. According to data we collected, the results are promising, as all these centers have requested further reinforcements from the Ministry of Health; which has asked us to produce extra quantities".
On the drug administration to home quarantined patients and the importance of medical supervision, Shuker said, "The drug is preferred to be taken after consulting a doctor and according to a prescription. However, it does not require medical supervision as this drug so far does not have side effects. If it is recommended by the doctor, the patient can quarantine himself at home and be assured; noting that the drug is not given to pregnant and nursing mothers".
Haidar Shuker added, "the patient should start treatment early after testing positive, as the goal of the treatment is to prevent the virus from multiplying. The Iraqi therapeutic protocol approved eight pills in the morning and eight other pills in the evening for the first day, followed by three pills in the morning and three in the evening for four or five days or according to the patient's response”.